Details of the Drug
General Information of Drug (ID: DM53BDW)
| Drug Name |
Trioxsalen
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
trioxsalen; 3902-71-4; Trioxysalen; Trisoralen; Trimethylpsoralen; 4,5',8-Trimethylpsoralen; Elder 8011; Trioxysalenum; Trioxisaleno; Trioxysalene; 2',4,8-Trimethylpsoralen; 4,5',8-Trimethylpsoralene; NSC-71047; 2,5,9-Trimethyl-7H-furo[3,2-g]chromen-7-one; UNII-Y6UY8OV51T; Trioxysalene [INN-French]; Trioxysalenum [INN-Latin]; NSC71047; Trioxisaleno [INN-Spanish]; NSC 71047; TRIOXSALIN; 7H-Furo[3,2-g][1]benzopyran-7-one, 2,5,9-trimethyl-; EINECS 223-459-0; 4,8,5'-Trimethylpsoralen; BRN 0221723; Y6UY8OV51T
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
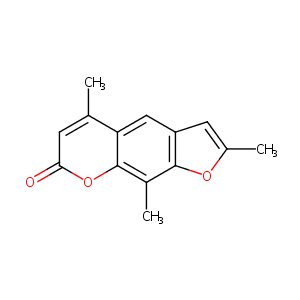 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
