| Drug Name |
N-desethyl sunitinib
|
| Synonyms |
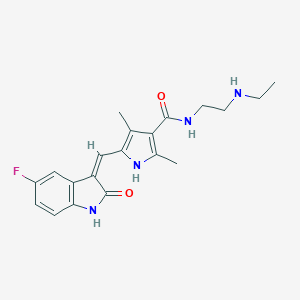
N-Desethyl Sunitinib; N-DesethylSunitinib; UNII-42LJ35612R; 42LJ35612R; C20H23FN4O2; N-[2-(ethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide; 5-(5-Fluoro-2-oxo-1,2-dihydro-indol-(3Z)-ylidenemethyl)-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (2-ethylamino-ethyl)-amide; 5-[5-fluoro-2-oxo-1,2-dihydro-indol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (2-ethylamino-ethyl)-amide; SCHEMBL2115189; CHEMBL3542344; DTXSID10437828
|
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
370.4 |
|
| Logarithm of the Partition Coefficient (xlogp) |
1.8 |
| Rotatable Bond Count (rotbonds) |
6 |
| Hydrogen Bond Donor Count (hbonddonor) |
4 |
| Hydrogen Bond Acceptor Count (hbondacc) |
4 |
| Chemical Identifiers |
- Formula
- C20H23FN4O2
- IUPAC Name
N-[2-(ethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide - Canonical SMILES
-
CCNCCNC(=O)C1=C(NC(=C1C)C=C2C3=C(C=CC(=C3)F)NC2=O)C
- InChI
-
InChI=1S/C20H23FN4O2/c1-4-22-7-8-23-20(27)18-11(2)17(24-12(18)3)10-15-14-9-13(21)5-6-16(14)25-19(15)26/h5-6,9-10,22,24H,4,7-8H2,1-3H3,(H,23,27)(H,25,26)/b15-10-
- InChIKey
-
LIZNIAKSBJKPQC-GDNBJRDFSA-N
|
| Cross-matching ID |
- PubChem CID
- 10292573
- CAS Number
-
- VARIDT ID
- DR01638
|
|
|
|
|
|
|
|



