Details of the Drug
General Information of Drug (ID: DM5EKPM)
| Drug Name |
B-581
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
149759-96-6; B581; FTase Inhibitor I; B 581; B-581; CHEMBL91722; CHEBI:83620; N-[2(S)-(2(R)-2-Amino-3-mercaptopropylamino)-3-methylbutyl]-L-phenylalanyl-L-methionine trifluoroacetate salt; (S)-2-((S)-2-((S)-2-((R)-2-amino-3-mercaptopropylamino)-3-methylbutylamino)-3-phenylpropanamido)-4-(methylthio)butanoic acid; CBiol_001875; H-Cys-psi(CH2NH)Val-psi(CH2NH)Phe-Met-OH; AC1L31RF; BSPBio_001466; KBioSS_000186; KBioGR_000186; SCHEMBL2723960; KBio3_000372; KBio2_005322; KBio2_000186; KBio3_000371; KBio2_002754; CTK8E9094
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
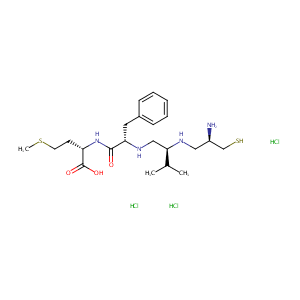 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 470.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 16 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
