Details of the Drug
General Information of Drug (ID: DM5OY70)
| Drug Name |
Iron Dextran
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Chinofer; Dexferrum; Dextrofer; Eisendextran; Fenate; Ferridextran; Ferrodextran; Ferroglucin; Hematran; Icar; Imfergen; Imferon; Imperon; Imposil; Infed; Norferan; Polyfer; Proferdex; Prolongal; Ursoferran; Dextran iron complex; Eisendextran [German]; Fero Gradumet; Ferric dextran; Iron dextran complex; Iron dextran injection; Iron hydrogenated dextran; Ironorm injection; B 75; Dextrofer 75; Ferdex 100; Ferroglukin 75; Myofer 100; A 100 (Pharmaceutical); American Regent Brand of Iron-Dextran Complex; Dextran-Iron Complex; Fe-Dextran; GlaxoSmithKline Brand of Iron-Dextran Complex; Goldline Brand of Iron-Dextran Complex; Hauck Brand of Iron-Dextran Complex; Hawthron Brand of Iron-Dextran Complex; Iro-Jex; Iron-dextran complex; Sanofi Brand of Iron-Dextran Complex; Sulfuric acid, iron salt; Sulphuric acid, iron salt; Vortech Brand of Iron-Dextran Complex; Watson Brand of Iron-Dextran Complex; Sulfuric acid iron salt (1:1); Sulfuric acid, iron salt (1:); Sulfuric acid iron(2+) salt (1:1)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Iron Supplement
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
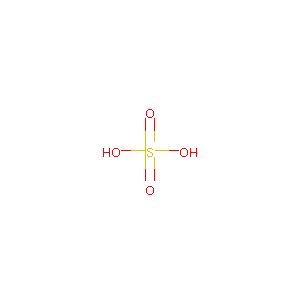 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 153.93 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Iron Dextran
|
|||||||||||||||||||||||||||||
References
