Details of the Drug
General Information of Drug (ID: DM64ZGY)
| Drug Name |
Vafidemstat
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Vafidemstat; 1357362-02-7; ORY-2001; Vafidemstat [INN]; LZ82JLT4UP; UNII-LZ82JLT4UP; 1,3,4-Oxadiazole-2-methanamine, 5-amino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; 5-((((1R,2S)-2-(4-(benzyloxy)phenyl)cyclopropyl)amino)methyl)-1,3,4-oxadiazol-2-amine; 5-[[[(1R,2S)-2-(4-phenylmethoxyphenyl)cyclopropyl]amino]methyl]-1,3,4-oxadiazol-2-amine; (1R,2S)-2-(4-(Benzyloxy)phenyl)-N-((5-imino-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)cyclopropanamine; 1,3,4-Oxadiazole-2-methanamine, 4,5-dihydro-5-imino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; SCHEMBL528204; CHEMBL4802155; XBBRLCXCBCZIOI-DLBZAZTESA-N; BCP29383; BDBM50594947; AKOS040742807; MS-25100; HY-112623; CS-0058593; ORY 2001; ORY-2001; ORY2001; A930244; 5-(((trans)-2-(4-(benzyloxy)phenyl)cyclopropylamino)methyl)-1,3,4-oxadiazol-2-amine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
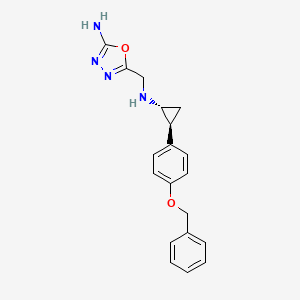 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Alzheimer disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A20 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
