Details of the Drug
General Information of Drug (ID: DM79E2K)
| Drug Name |
Aminodeoxykanamycin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aminodeoxykanamycin; bekanamycin; Kanamycin B; nebramycin factor 5; Bekanamycine; Bekanamycinum; Becanamicina; Bekanamycinum [INN-Latin]; Bekanamycine [INN-French]; Becanamicina [INN-Spanish]; EINECS 225-170-5; BRN 0061646; Aminodeoxykanamycin sulfate; 4696-76-8; Antibiotic derived from Streptomyces kanamyceticus; AC1L2FQA; AC1Q57UP; Antibiotic derived from Streptomyces kanamyceticus. Kanamycin B; (1r,2s,4r,6s)-4,6-diamino-3-[(3-amino-3-deoxy-; A-d-glucopyranosyl)oxy]-2-hydroxycyclohexyl 2,6-diamino-2,6-dideoxy-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
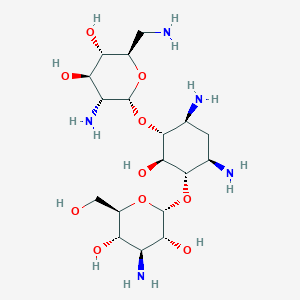 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 483.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -7.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 11 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 15 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
