Details of the Drug
General Information of Drug (ID: DM79KLR)
| Drug Name |
nafadotride
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Nafadotride; 149649-22-9; (+-)-nafadotride; N-[(1-BUTYL-2-PYRROLIDINYL)METHYL]-4-CYANO-1-METHOXY-2-NAPHTHALENECARBOXAMIDE; N-[(1-butylpyrrolidin-2-yl)methyl]-4-cyano-1-methoxynaphthalene-2-carboxamide; CHEMBL286252; CHEBI:64191; A1-01951; SMR000466292; AC1MPBUT; GTPL44; MLS000758952; MLS001424218; SCHEMBL635866; DTXSID1042603; CTK8H0240; MolPort-023-276-076; HMS3394A15; HMS3267P15; HMS2232B11; HMS2052A15; HMS3370D02; BCP27779; BDBM50133923; AKOS024257976; TRA0072321; CCG-101119; NC00369; SAM001247092; CPD000466292; LS-186981; LS-187623
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
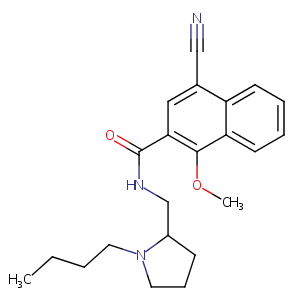 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 365.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
