Details of the Drug
General Information of Drug (ID: DM7OHSX)
| Drug Name |
2-phenylpiperidine hydrochloride
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
2-Phenylpiperidine; 3466-80-6; 2-phenyl-piperidine; Piperidine, 2-phenyl-; 2-phenyl piperidine; EINECS 222-422-6; PubChem8019; (Piperidin-2-yl)benzene; ACMC-1AG1G; AC1L2U2P; AC1Q1H0F; SCHEMBL938781; SCHEMBL11523711; CTK1C4792; MolPort-000-147-784; WGIAUTGOUJDVEI-UHFFFAOYSA-N; KS-00000N7T; ALBB-006245; SBB022661; STK312087; BBL022420; AKOS000184053; AKOS016344001; FS-1688; MCULE-8351203351; ACN-027346; KB-84880; AM803744; SC-48762; TR-014336; ST2404539; AB0089977; ST45091094; P2005; BB 0254270; A6090; FT-0656912; CS-0028391; K-7884; J-019700
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
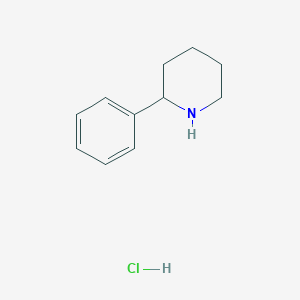 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 197.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
