Details of the Drug
General Information of Drug (ID: DM83951)
| Drug Name |
AG-E-85378
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
N-(4-Chlorophenyl)-2-[(pyridin-4-ylmethyl)amino]benzamide; 269390-69-4; VEGFR Tyrosine Kinase Inhibitor II; VEGF Receptor Tyrosine Kinase Inhibitor II; CHEMBL101683; 3hng; AG-E-85378; ZINC8732; SCHEMBL3005186; GTPL6056; N-(4-chlorophenyl)-2-(pyridin-4-ylmethylamino)benzamide; CTK4F8762; DTXSID60430900; MolPort-044-561-457; HMS3229O07; IN1017; BDBM50132151; AKOS030580576; DB07288; CCG-206804; NCGC00387770-01; ACM269390694; RT-016223; KB-299355; J-016613; N-(4-Chlorophenyl)-2-(4-pyridinylmethylamino)benzamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
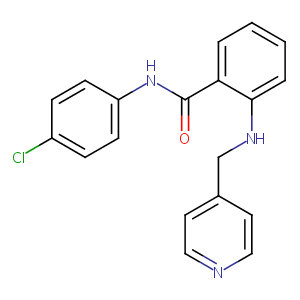 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 337.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
