Details of the Drug
General Information of Drug (ID: DM8IC5H)
| Drug Name |
TRICIRIBINE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
NSC154020; Akt Inhibitor V, Triciribine; MLS002702033; Akt/PKB Signaling Inhibitor-2; NSC-154020; TCN; Akt inhibitor V; AC1Q7CGY; AC1L6DVE; tricyclic nucleoside (TCN); SCHEMBL61269; GTPL5920; CHEMBL1892348; CHEBI:91697; HMS3268N05; HMS3654C06; HMS3229A09; HSCI1_000386; CCG-206732; 5-methyl-1-pentofuranosyl-1,5-dihydro-1,4,5,6,8-pentaazaacenaphthylen-3-amine; SMR001565606; SC-89664; NCI60_001091; Akt/protein kinase B signaling inhibitor-2; BRD-A42649439-001-01-0; BRD-A42649439-001-02-8; 1,5,6,8-Pentaazaacennaphthylen-3-amine, 1,5-dih
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
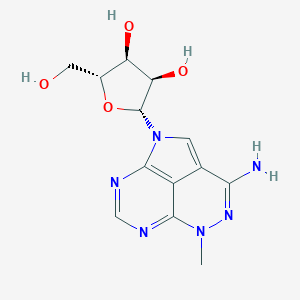 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 320.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
References
