Details of the Drug
General Information of Drug (ID: DM8J0MK)
| Drug Name |
GEA-6414
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Tolfenamic acid; Acide tolfenamique; Acido tolfenamico; Acidum tolfenamicum; Bifenac; Clotam; GEA 6414; Tolfedine; tolfenamic acid; 13710-19-5; 2-(3-Chloro-2-methylanilino)benzoic acid; 2-[(3-Chloro-2-methylphenyl)amino]benzoic Acid; 3G943U18KM; Anthranilic acid, N-(3-chloro-o-tolyl)-; Benzoic acid, 2-[(3-chloro-2-methylphenyl)amino]-; CAS-13710-19-5; CHEBI:32243; N-(2-Methyl-3-chlorophenyl)anthranilic acid; N-(3-Chloro-2-methylphenyl)anthranilic acid; N-(3-Chloro-o-tolyl)-anthranilic acid; NCGC00016705-05; UNII-3G943U18KM
|
|||||
| Affected Organisms |
Humans and other mammals
|
|||||
| ATC Code |
|
|||||
| Structure |
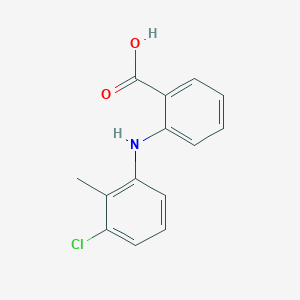 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 261.7 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 5.2 | |||||
| Rotatable Bond Count (rotbonds) | 3 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
References
