Details of the Drug
General Information of Drug (ID: DM8W4N9)
| Drug Name |
Apratastat
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
TMI-005; TMI-05; UNII-C6BZ5263BJ; 287405-51-0; C6BZ5263BJ; CHEMBL206815; TMI 005; Apratastat [USAN:INN]; Apratastat (USAN/INN); MLS006010301; SCHEMBL2834310; GTPL6482; TMI005; Apratastat, > MolPort-021-805-014; BCPP000041; ZINC28571311; BDBM50181008; DB13020; API0013699; compound 5h [PMID: 16426848]; SMR004701369; 4CA-0170; D08859; 3-Thiomorpholinecarboxamide,N-hydroxy-4-[[4-[(4-hydroxy-2-butyn-1-yl)oxy]phenyl]sulfonyl]-2,2-dimethyl-,(3S)-; TMI-1; Dual TACE/MMP-13 inhibitors (inflammation), Wyeth; Dual TACE/MMP-13 inhibitors (rheumatoid arthritis), Wyeth; Dual TACE/MMP-13 inhibitors, Wyeth-Ayerst
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
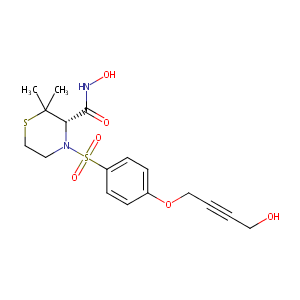 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 414.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
