Details of the Drug
General Information of Drug (ID: DM9R1YU)
| Drug Name |
Gestodene
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Gestinol; Gestoden; Gestodene [USAN:INN:BAN]; Gestodeno; Gestodeno [INN-Spanish]; Gestodenum; Gestodenum [INN-Latin]; SH B 331; GESTODENE; 13-Ethyl-17-hydroxy-18,19-dinor-17alpha-pregna-4,15-dien-20-yn-3-one; 1664P6E6MI; 17-alpha-Ethinyl-13-ethyl-17-beta-hydroxy-4,15-gonadien-3-one; 18,19-Dinorpregna-4,15-dien-20-yn-3-one, 13-ethyl-17-hydroxy-, (17-alpha)-; 60282-87-3; BRN 4237181; CCRIS 9189; DSSTox_CID_26478; DSSTox_GSID_46478; DSSTox_RID_81649; EINECS 262-145-8; HSDB 3594; UNII-1664P6E6MI
|
|||||
| Structure |
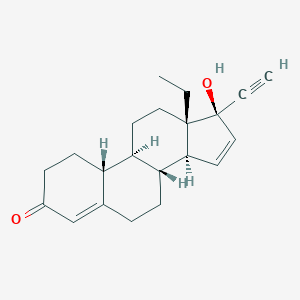 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 310.4 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | |||||
| Rotatable Bond Count (rotbonds) | 2 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
