Details of the Drug
General Information of Drug (ID: DM9U1HV)
| Drug Name |
5-fluoro-1H-indole-2-carboxylic acid
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
5-Fluoroindole-2-carboxylic acid; 399-76-8; 5-Fluoro-1H-indole-2-carboxylic acid; 2-Carboxy-5-fluoroindole; 1H-Indole-2-carboxylic acid, 5-fluoro-; CHEMBL23507; MLS000080089; WTXBRZCVLDTWLP-UHFFFAOYSA-N; MFCD00005612; SMR000037735; 5-Fluoroindole-2-carboxylic acid, 98%; Spectrum_001495; EINECS 206-919-5; PubChem1683; SpecPlus_000678; Spectrum5_001733; Opera_ID_1340; Spectrum4_001182; Spectrum3_001043; Spectrum2_001469; ACMC-209j9k; AC1Q4NE3; Lopac-265128; cid_1820; AC1Q73TI; AC1L1CB9; AC1Q73TJ; Lopac0_000071; Oprea1_012690
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
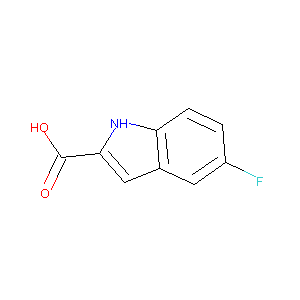 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 179.15 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.4 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
