Details of the Drug
General Information of Drug (ID: DM9WHMF)
| Drug Name |
PF-07081532
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Lotiglipron; PF-07081532; 2401892-75-7; LOTIGLIPRON [USAN]; YI10W1K93A; SCHEMBL22009794; BDBM450633; EX-A7734; US10676465, Example 10; HY-153865; CS-0865718; 1H-Benzimidazole-6-carboxylic acid, 2-[[4-[(2S)-2-(5-chloro-2-pyridinyl)-2-methyl-1,3-benzodioxol-4-yl]-1-piperidinyl]methyl]-1-[(2S)-2-oxetanylmethyl]-; 2-({4-[(S)-2-(5-Chloropyridin-2-yl)-2-methylbenzo[1,3]dioxol-4-yl]piperidin-1-yl}methyl)-1-{[(S)-oxetan-2-yl]methyl}-1H-benzimidazole-6-carboxylic acid
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||||||
| Structure |
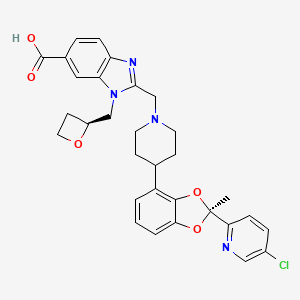 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Obesity | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5B81 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
