Details of the Drug
General Information of Drug (ID: DMAR467)
| Drug Name |
Xylose
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
D-Xylose; D-Xylopyranose; Xyloside; D-xylopentose; (3R,4S,5R)-oxane-2,3,4,5-tetrol; 7261-26-9; CHEBI:53455; Xylopyranoside; D-Xylopyranoside; Xylomed; D-Xyl; 10257-31-5; (3R,4S,5R)-tetrahydro-2H-pyran-2,3,4,5-tetrol; (3R,4S,5R)-Tetrahydro-2H-pyran-2,3,4,5-tetraol; D-(+)-Xylose, > Xyl; Xylose (USP); Xylo-Pfan (TN); DSSTox_CID_3745; Epitope ID:114701; AC1L35YW; DSSTox_RID_77180; SCHEMBL39891; DSSTox_GSID_23745; MLS001361339; cid_135191; CHEMBL502135; DTXSID0023745; CTK0H1735; BDBM16234; MolPort-003-934-029; D-xylose
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
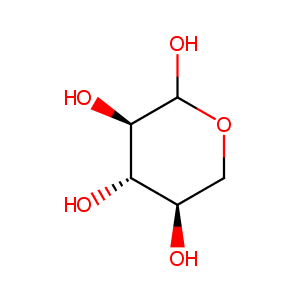 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 150.13 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
