Details of the Drug
General Information of Drug (ID: DMAYTRK)
| Drug Name |
H3B-6527
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
MBWRLLRCTIYXDW-UHFFFAOYSA-N; 1702259-66-2; UNII-4HTE364XIK; 4HTE364XIK; N-[2-[[6-[(2,6-dichloro-3,5-dimethoxyphenyl)carbamoyl-methylamino]pyrimidin-4-yl]amino]-5-(4-ethylpiperazin-1-yl)phenyl]prop-2-enamide; Eisai/H3 Biomedicine; CHEMBL3939295; SCHEMBL16659467; BDBM249396; EX-A1510; BCP17555; ZINC521836463; AKOS032960479; H3B6527; CS-5830; ACN-037543; AK684091; AC-29878; HY-100491; US9434697, 108; N-(2-((6-(3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-methylureido)pyrimidin-4-yl)amino)-5-(4-ethylpiperazin-1-yl)phenyl)acryla
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
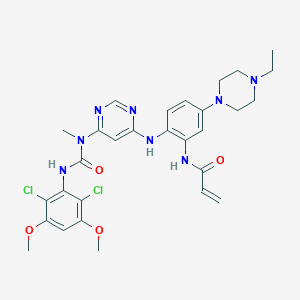 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 629.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
