Details of the Drug
General Information of Drug (ID: DMBDW8P)
| Drug Name |
3-(4-sulfamoylphenyl)propanoic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
3-(4-Sulfamoylphenyl)propanoic acid; 90610-69-8; 3-(4-Sulfamoyl-phenyl)-propionic acid; 3-[4-(Aminosulfonyl)phenyl]propanoic Acid; CHEMBL451332; 4-(2-carboxyethyl)-benzenesulfonamide; M28; BAS 12384209; AC1MKR5Y; AC1Q55BB; 4-carboxyethylbenzenesulfonamide; SCHEMBL4964212; CTK7J3040; BDBM29277; MolPort-001-769-253; JUEONDBIBADVGD-UHFFFAOYSA-N; HMS3604J11; ZINC4362893; 4-(Aminosulphonyl)hydrocinnamic acid; STK802680; ANW-44559; SBB011591; BBL002635; 4-(2-Carboxyethyl)benzenesulphonamide; P-CARBOXYETHYLBENZENESULFONAMIDE; 3-[4-(AMINOSULFONYL)PHENYL]PROPANOIC ACID
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
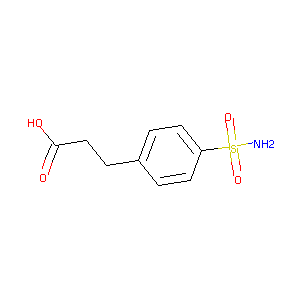 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 229.26 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
