Details of the Drug
General Information of Drug (ID: DMBXVMZ)
| Drug Name |
Dihydroartemisinin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Artenimol; Cotecxin; Dihydroartemisinine; Dihydroqinghaosu; Dihydroquinghaosu; Dihydroquinghoasu; VM3352; Artenimol (INN); JAV-110; GNF-PF-5634; (3R,5aS,6R,8aS,9R,12R,12aR)-3,6,9-trimethyldecahydro-3,12-epoxypyrano[4,3-j][1,2]benzodioxepin-10-ol; [3R-(3.alpha.,5a.beta.,6.beta.,8a.beta.,9.alpha.,10.alpha.,12.beta.,12aR*)]-Decahydro-10-hydroxy-3,6,9-trimethyl-3,12-epoxy-12H-pyrano[4.3-j]-1,2-benzodioxepin; 1,5,9-trimethyl-(1R,4S,5R,8S,9R,10S,12R,13R)-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecan-10-ol(Dihydroartemisinin)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
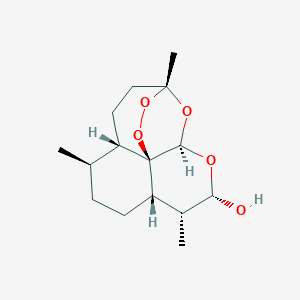 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 284.35 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
