Details of the Drug
General Information of Drug (ID: DMCAR7B)
| Drug Name |
O-Sialic Acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
alpha-Neu5Ac; N-acetyl-alpha-neuraminic acid; O-sialic acid; CHEBI:49026; N-Acetyl-a-neuraminic acid; UNII-04A90EXP8V; sialic acid; 04A90EXP8V; N-acetyl-alpha-neuraminate; NANA; 5-acetamido-3,5-dideoxy-D-glycero-alpha-D-galacto-non-2-ulopyranosonic acid; 131-48-6; SIA; (2R,4S,5R,6R)-5-acetamido-2,4-dihydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid; polysialic acid; Alpha-Sialoside; 4lkh; 2qwb; N-Acetyl-a-neuraminate; N-Acetyl-a-D-neuraminate; Epitope ID:136794; SCHEMBL79085; N-Acetyl-a-D-neuraminic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
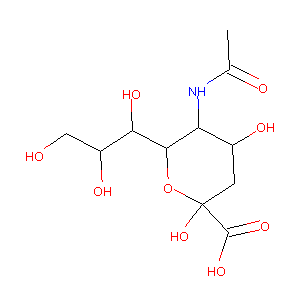 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 309.27 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -3.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
