Details of the Drug
General Information of Drug (ID: DMCEZ1B)
| Drug Name |
Cotinine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
cotinine; (-)-Cotinine; 486-56-6; Cotinina; Cotininum; (S)-(-)-Cotinine; (5S)-1-methyl-5-(pyridin-3-yl)pyrrolidin-2-one; Cotinine (-); (S)-Cotinine; UNII-K5161X06LL; (S)-1-Methyl-5-(3-pyridinyl)-2-pyrrolidinone; 2-Pyrrolidinone, 1-methyl-5-(3-pyridinyl)-, (5S)-; CHEBI:68641; (S)-1-Methyl-5-(3-pyridyl)-2-pyrrolidinone; UIKROCXWUNQSPJ-VIFPVBQESA-N; K5161X06LL; Cotinine [INN]; MFCD00077696; Cotininum [INN-Latin]; Cotinina [INN-Spanish]; S(-)-1-Methyl-5-(3-pyridyl)-2-pyrrolidone; (5S)-1-methyl-5-pyridin-3-ylpyrrolidin-2-one
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
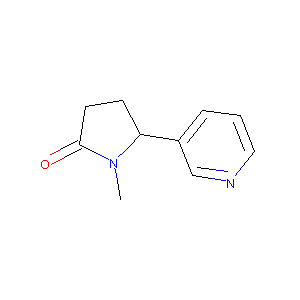 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 176.21 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
