Details of the Drug
General Information of Drug (ID: DMCVY4U)
| Drug Name |
L-689560
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
trans-2-Carboxy-5,7-dichloro-4-(((phenylamino)carbonyl)amino)-1,2,3,4-tetrahydroquinoline; C17H15Cl2N3O3; L 689560; L-689,560; 4-trans-2-carboxy-5,7-dichloro-4-phenylaminocarbonylamino-1,2,3,4-tetrahydroquinoline; 139051-78-8; (2S,4R)-5,7-dichloro-4-(phenylcarbamoylamino)-1,2,3,4-tetrahydroquinoline-2-carboxylic acid; 2-Quinolinecarboxylic acid, 5,7-dichloro-1,2,3,4-tetrahydro-4-(((phenylamino)carbonyl)amino)-, trans-(+-)-; [3H]L689560; AC1L3TN7; SCHEMBL193615; GTPL4239; GTPL4086; ZINC2567729; compound 35
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
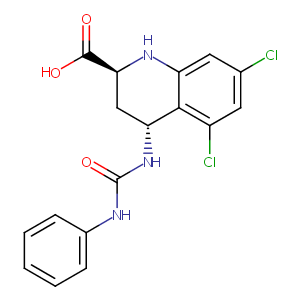 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 380.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
References
