Details of the Drug
General Information of Drug (ID: DMDC297)
| Drug Name |
(R)-1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
(R)-1-(4-Iodo-2,5-dimethoxyphenyl)propan-2-amine; CHEMBL134519; R-DOI; Lopac0_000478; GTPL157; (R)-DOI; SCHEMBL713061; ZINC2516053; PDSP2_001372; Benzeneethanamine, 4-iodo-2,5-dimethoxy-alpha-methyl-, (alphaR)-; BDBM50133231; PDSP1_001388; CCG-204569; NCGC00162167-01; AJ-36945; 82864-06-0; (2R)-1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine; (r)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane; (R)-2-(4-iodo-2,5-dimethoxyphenyl)-1-methylethylamine; (R)-2-(4-Iodo-2,5-dimethoxy-phenyl)-1-methyl-ethylamine; UNII-OOM10GW9UE
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
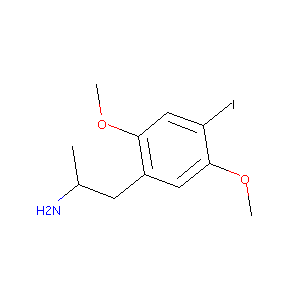 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 321.15 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
