Details of the Drug
General Information of Drug (ID: DMDGPKS)
| Drug Name |
RG7625
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Petesicatib; UNII-A26QO95U37; A26QO95U37; RG-7625; RO-5459072; Petesicatib [INN]; SCHEMBL700776; GTPL9855; KXAAIORSMACJSI-AEFFLSMTSA-N; RO5459072; 2-Pyrrolidinecarboxamide, N-(1-cyanocyclopropyl)-4-((4-(1-methyl-1H-pyrazol-4-yl)-2-(trifluoromethyl)phenyl)sulfonyl)-1-((1-(trifluoromethyl)cyclopropyl)carbonyl)-, (2S,4R)-; 1252637-35-6; (2S,4R)-N-(1-cyanocyclopropyl)-4-[4-(1-methylpyrazol-4-yl)-2-(trifluoromethyl)phenyl]sulfonyl-1-[1-(trifluoromethyl)cyclopropanecarbonyl]pyrrolidine-2-carboxamide; (2s,4r)-4-[4-(1-me
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Structure |
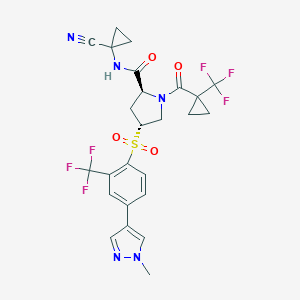 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 603.5 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.6 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 12 | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
