Details of the Drug
General Information of Drug (ID: DME3FUD)
| Drug Name |
2-(2,4-dichlorophenoxy)-5-(3-phenylpropyl)phenol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
2-(2,4-dichlorophenoxy)-5-(3-phenylpropyl)phenol; JPN; Triclosan derivative, 22; CHEMBL259880; BDBM25421; 6760-EP2316452A1; 6760-EP2311801A1; 6760-EP2311797A1; 6760-EP2311455A1; 6760-EP2301536A1; 6760-EP2298767A1; 6760-EP2281818A1; 6760-EP2311798A1; 6760-EP2308509A1; 6760-EP2305250A1; 6760-EP2301627A1; 6760-EP2301538A1; 6760-EP2298734A2; 6760-EP2295053A1; 6760-EP2292614A1; 6760-EP2287155A1; 6760-EP2277876A1; 6760-EP1441224A2; 6760-EP2314587A1; 6760-EP2311831A1; 6760-EP2311799A1; 6760-EP2308874A1; 6760-EP2308873A1; 6760-EP2305653A1
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
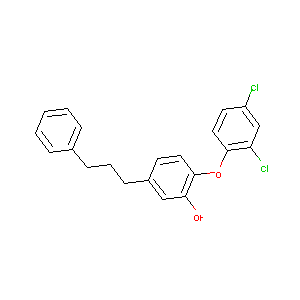 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 373.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 7.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
