| Drug Name |
Pyrene-1-aldehyde
|
| Synonyms |
Pyrene-1-carbaldehyde; RCYFOPUXRMOLQM-UHFFFAOYSA-N; 1-Formylpyrene; KSC224M2B; PYRENECARBOXALDEHYDE; pyrene carboxaldehyde; pyrene-1-carboxaldehyde; pyrenecarbaldehyde; 1-PYRENECARBOXALDEHYDE; 1-Pyrene carboxaldehyde; 1-Pyrene-carboxaldehyde; 1-Pyrenealdehyde; 1-Pyrenecarbaldehyde; 3-Formylpyrene; 3-Pyrenealdehyde; 3-Pyrenecarboxaldehyde; 3-Pyrenylaldehyde; 3029-19-4; AC1L5OS9; AC1Q6PUX; AC1Q6QH9; ACMC-209hea; CCRIS 3163; EINECS 221-196-6; I9H95PVI1P; MFCD00004139; NSC 30811; SCHEMBL52121; UNII-I9H95PVI1P
|
| Structure |
|
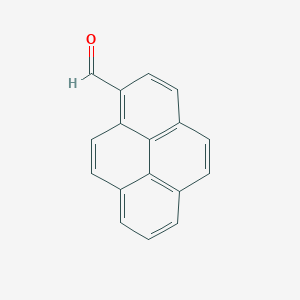 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
230.26 |
|
| Logarithm of the Partition Coefficient (xlogp) |
4.5 |
| Rotatable Bond Count (rotbonds) |
1 |
| Hydrogen Bond Donor Count (hbonddonor) |
0 |
| Hydrogen Bond Acceptor Count (hbondacc) |
1 |
| Chemical Identifiers |
- Formula
- C17H10O
- IUPAC Name
pyrene-1-carbaldehyde - Canonical SMILES
-
C1=CC2=C3C(=C1)C=CC4=C(C=CC(=C43)C=C2)C=O
- InChI
-
RCYFOPUXRMOLQM-UHFFFAOYSA-N
- InChIKey
-
1S/C17H10O/c18-10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)17(13)16(11)12/h1-10H
|
| Cross-matching ID |
- PubChem CID
- 232848
- CAS Number
-
- INTEDE ID
- DR2002
|
|
|
|
|
|
|
|

