Details of the Drug
General Information of Drug (ID: DMEPWYA)
| Drug Name |
THIOCOLCHICOSIDE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Thiocolchicoside; 602-41-5; Coltramyl; UNII-T1X8S697GT; Thiocolchicine 2-glucoside analog; NSC 147755; Musco-ril; T1X8S697GT; 10-Thiocolchicoside; Tiocolchicoside [DCIT]; Colchicoside, 10-thio-; Thiocolchicoside [INN:DCF]; Q-201823; Thiocolchicosidum [INN-Latin]; Tiocolchicosido [INN-Spanish]; C27H33NO10S; Tiocolchicosido; Tiocolchicoside; Muscoril; Thiocolchicosidum; EINECS 210-017-7; BRN 0072205; R. 271; 2-10-Di(demethoxy)-2-glucosyloxy-10-methylthiocolchicine; Miotens; Coltrax; NSC147755; Coltramyl (TN); NCGC00016519-01
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
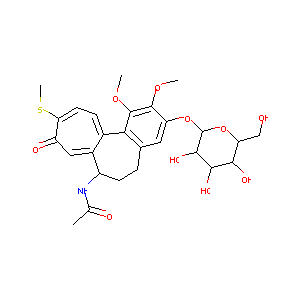 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 563.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
References
