Details of the Drug
General Information of Drug (ID: DMF8B62)
| Drug Name |
Linetastine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tmk688; UNII-7U248Z56LA; 110501-66-1; TMK-688; 159776-68-8; 7U248Z56LA; Linetastine [INN]; Tmk 688; Linazolast; CCRIS 6902; Linazolast (JAN); YM-257; AC1O5R98; CHEMBL314338; SCHEMBL1614484; [4-[(1E,3E)-5-[2-(4-benzhydryloxypiperidin-1-yl)ethylamino]-5-oxopenta-1,3-dienyl]-2-methoxyphenyl] ethyl carbonate; Carbonic acid, 4-(5-((2-(4-(diphenylmethoxy)-1-piperidinyl)ethyl)amino)-5-oxo-1,3-pentadienyl)-2-methoxyphenyl ethyl ester; D09850; Linazolast; Molecule 26
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
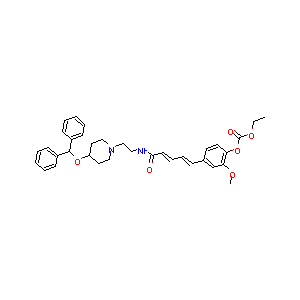 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 584.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 15 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Rhinitis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | FA20 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
