Details of the Drug
General Information of Drug (ID: DMFEHXD)
| Drug Name |
Natamycin
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Delvocid; Delvolan; Delvopos; Mycophyt; Myprozine; Natacyn; Natafucin; Natamicina; Natamycine; Natamycinum; Pimafucin; Pimaricin; Pimaricine; Pimarizin; Synogil; Tennecetin; Natamycin preparation; Pimaricin preparation; Pimarizin [German]; CL 12625; Antibiotic A-5283; CL 12,625; CL-12625; Natacyn (TN); Natamicina [INN-Spanish]; Natamycine [INN-French]; Natamycinum [INN-Latin]; Pimaricin (JP15); Natamycin (USP/INN); Natamycin [USAN:INN:BAN]; Stereoisomer of 22-((3-amino-3,6-dideoxy-beta-D-mannopyranosyl)oxy)-1,3,26-trihydroxy-12-methyl-10-oxo-6,11,28-trioxatricyclo(22.3.1.0(sup 5,7))octacosa-8,14,16,18,20-pentaene-25-carboxylic acid; (1R,3S,5R,7R,8E,12R,14E,16E,18E,20E,22R,24S,25R,26S)-22-[(3-amino-3,6-dideoxy-beta-D-mannopyranosyl)oxy]-1,3,26-trihydroxy-12-methyl-10-oxo-6,11,28-trioxatricyclo[22.3.1.0~5,7~]octacosa-8,14,16,18,20-pentaene-25-carboxylic acid; 16-(3-Amino-3,6-didesoxy-beta-D-mannopyranosyloxy)-5,6-epoxy-8,12,14-trihydroxy-26-methyl-2,10-dioxo-1-oxacyclohexacosa-3,17,19,21,23-pentaen-13-carbonsaeure; 6,11,28-Trioxatricyclo[22.3.1.05,7]octacosa-8,14,16,18,20-pentaene-25-carboxylic acid, 22-[(3-amino-3,6-dideoxy-.beta.-D-mannopyranosyl)oxy]-1,3,26-trihydroxy-12-methyl-10-oxo-, (1R,3S,5R,7R,8E,12R,14
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antifungal Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Various Fungus Species
|
||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
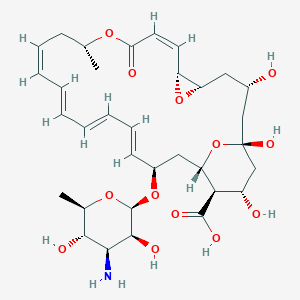 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 665.7 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.3 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 7 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 14 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
