Details of the Drug
General Information of Drug (ID: DMG0OUD)
| Drug Name |
GS4071
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Oseltamivir acid; 187227-45-8; Oseltamivir carboxylate; Oseltamivir (acid); GS 4071; Ro 64-0802; UNII-K6106LV5Q8; CHEMBL674; CHEBI:73139; K6106LV5Q8; GS-4071; (3R,4R,5S)-4-acetamido-5-amino-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylic acid; 5-N-ACETYL-3-(1-ETHYLPROPYL)-1-CYCLOHEXENE-1-CARBOXYLIC ACID; 1-Cyclohexene-1-carboxylic acid, 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-, (3R,4R,5S)-; (3r,4r,5s)-4-(Acetylamino)-5-Amino-3-(Pentan-3-Yloxy)cyclohex-1-Ene-1-Carboxylic Acid; G39; Oseltamivir-carboxylate; 2,4-diamino triazine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
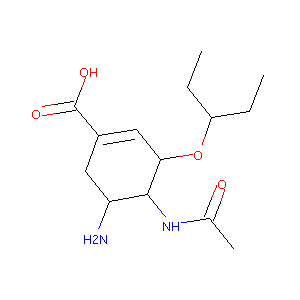 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 284.35 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
