Details of the Drug
General Information of Drug (ID: DMG21PZ)
| Drug Name |
Di(2,6-diisopropylphenol)
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
3,3',5,5'-Tetraisopropylbiphenyl-4,4'-diol; 2416-95-7; Dipropofo; UNII-H9GE6HX42A; di(2,6-diisopropylphenol); H9GE6HX42A; CHEMBL478518; 3,3',5,5'-Tetrakis(1-methylethyl)biphenyl-4,4'-diol; AK-51092; 3,3',5,5'-Tetraisopropyl-[1,1'-biphenyl]-4,4'-diol; Propofol related compound A; Dipropofol; Propofol related compound A [USP]; Propofol specified impurity E [EP]; Propofol Impurity E; Propofol related compound A RS [USP]; 3,3',5,5'-Tetraisopropyl-4,4'-dihydroxybiphenyl; SCHEMBL275427; CTK4F3066; DTXSID30178867
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
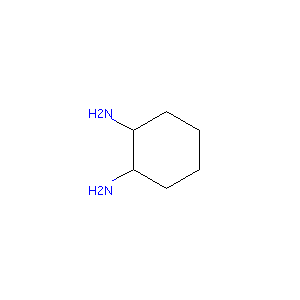 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 354.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 7.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
