Details of the Drug
General Information of Drug (ID: DMH3DXY)
| Drug Name |
FK-453
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
FK-453; FK 453; CHEMBL440115; 121524-18-3; (+)-(R)-((E)-3-(2-Phenylpyrazolo(1,5-a)pyridin-3-yl)acryloyl)-2-piperidineethanol; (R-(E))-1-(1-Oxo-3-(2-phenylpyrazolo(1,5-a)pyridin-3-yl)-2-propenyl)-2-piperidineethanol; C23H25N3O2; 2-Piperidineethanol, 1-(1-oxo-3-(2-phenylpyrazolo(1,5-a)pyridin-3-yl)-2-propenyl)-, (R-(E))-; (E)-1-[(2R)-2-(2-hydroxyethyl)piperidin-1-yl]-3-(2-phenylpyrazolo[1,5-a]pyridin-3-yl)prop-2-en-1-one; AC1O5QYH; SCHEMBL2826910; GTPL5606; SCHEMBL3074643; FK453; ZINC1486608; FR-453; BDBM50079652; FR113453
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
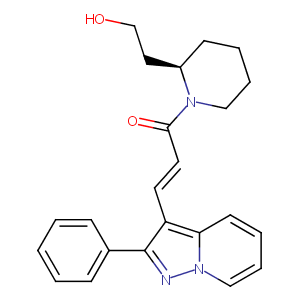 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 375.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
