Details of the Drug
General Information of Drug (ID: DMH6IKO)
| Drug Name |
Lusutrombopag
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | LUSUTROMBOPAG; mulpleta; UNII-6LL5JFU42F; 6LL5JFU42F; 2-Propenoic acid, Lusutrombopag [USAN:INN]; SCHEMBL3062 | ||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Structure |
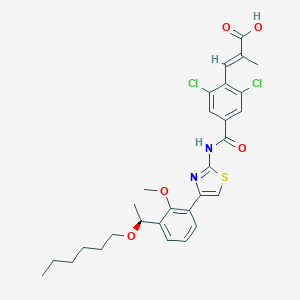 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 591.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 7.7 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 13 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 03 Disease of the blood or blood-forming organs | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: 3B64 Thrombocytopenia | |||||||||||||||||||||||
| The Studied Tissue | Whole blood | |||||||||||||||||||||||
| The Studied Disease | Thrombocytopenia [ICD-11:3B64] | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Lusutrombopag (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | 2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89. | ||||
|---|---|---|---|---|---|
| 2 | ClinicalTrials.gov (NCT01054443) A Study to Investigate the Efficacy and Safety of S-888711 Tablets Administered to Adult Subjects With Immune Thrombocytopenia (ITP). U.S. National Institutes of Health. | ||||
| 3 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 4 | Cerner Multum, Inc. "Australian Product Information.". | ||||
