Details of the Drug
General Information of Drug (ID: DMHA18P)
| Drug Name |
Lasofoxifene
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Oporia; 180916-16-9; rac-Lasofoxifene; CP 336156; Fablyn; UNII-337G83N988; (5R,6S)-6-phenyl-5-[4-(2-pyrrolidin-1-ylethoxy)phenyl]-5,6,7,8-tetrahydronaphthalen-2-ol; CHEMBL328190; 337G83N988; (-)-cis-5,6,7,8-Tetrahydro-6-phenyl-5-(p-(2-(1-pyrrolidinyl)ethoxy)phenyl)-2-naphthol; 180915-78-0; CP-336,156; LASOFOXIFENE HCL; Lasofoxifene [INN:BAN]; AC1L50OI; SCHEMBL26815; GTPL7542; CTK8F1062; BDBM20606; DTXSID50171037; GXESHMAMLJKROZ-IAPPQJPRSA-N; ZINC3918428; BCP03626; AKOS030241621; AN-3516; BCP9000842; DB06202; Oporia; Lasofoxifene [INN]; Cis-1R-(4'-pyrrolidinoethoxyphenyl)-2S-phenyl-6-hydroxy-1,2,3,4-tetrahydronaphthalene, tartrate salt; AZD9639
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
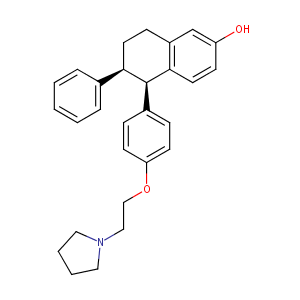 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 413.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.1 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
