Details of the Drug
General Information of Drug (ID: DMHELFU)
| Drug Name |
BF-389
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Biofor 389; BF-389; UNII-MGJ3XBS5KD; MGJ3XBS5KD; BF 389; 127245-22-1; 4-(3,5-Di-tert-butyl-4-hydroxybenzylidene)-2-methyl-5,6-dihydro-2H-1,2-oxazin-3(4H)-one; Dihydro-4-(3,5-di-tert-butyl-4-hydroxybenzylidene)-2-methyl-2H-1,2-oxazin-3(4H)-one; 2H-1,2-Oxazin-3(4H)-one, dihydro-4-((3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl)methylene)-2-methyl-; 2H-1,2-Oxazin-3(4H)-one, 4-((3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl)methylene)dihydro-2-methyl-; Biofor-389; AC1O5RBB; SCHEMBL8291651; LS-100029
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
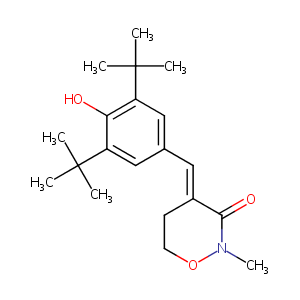 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 331.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Rheumatoid arthritis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | FA20 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
