Details of the Drug
General Information of Drug (ID: DMHP6OY)
| Drug Name |
AZD2066
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
AZD-2066; UNII-MQ908Y1ZB2; MQ908Y1ZB2; 934282-55-0; AZD 2066; AZD2066; SXWHYTICXCLKDG-GFCCVEGCSA-N; SCHEMBL1848325; CHEMBL3545164; MolPort-042-624-538; ZINC34885049; AKOS027470229; DB12644; Pyridine, 4-(5-((1R)-1-(5-(3-chlorophenyl)-3-isoxazolyl)ethoxy)-4-methyl-4H-1,2,4-triazol-3-yl)-; J3.560.339E; 5-(3-chlorophenyl)-3-[(1r)-1-[[4-methyl-5-(4-pyridyl)-1,2,4-triazol-3-yl]oxy]ethyl]isoxazole; 4-[5-[(1R)-1-[5-(3-Chlorophenyl)-3-isoxazolyl]ethoxy]-4-methyl-4H-1,2,4-triazol-3-yl]pyridine
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
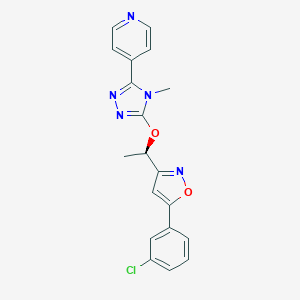 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 381.8 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
