Details of the Drug
General Information of Drug (ID: DMHPD2Y)
| Drug Name |
SODIUM CITRATE
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Sodium citrate; 68-04-2; TRISODIUM CITRATE; Citrosodine; Sodium citrate anhydrous; Citrosodina; Natrocitral; Citrosodna; Citreme; Citnatin; sodium citrate, anhydrous; Sodium 2-hydroxypropane-1,2,3-tricarboxylate; Trisodium citrate anhydrous; Citric acid, trisodium salt; Trisodium citrate, anhydrous; Citric acid trisodium salt; 1,2,3-Propanetricarboxylic acid, 2-hydroxy-, trisodium salt; UNII-RS7A450LGA; FEMA No. 3026; CCRIS 3293; Sodium citrate (Na3C6H5O7); HSDB 5201; EINECS 200-675-3; RS7A450LGA; CHEBI:53258; Trisodium 2-hydroxy-1
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
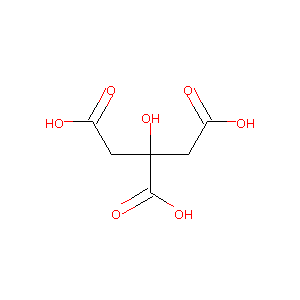 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 258.07 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count | 2 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count | 1 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 7 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Common cold | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA00 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from SODIUM CITRATE (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
References
