Details of the Drug
General Information of Drug (ID: DMHVL0J)
| Drug Name |
Azelaic Acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
AZA; Azelaic; Azelex; Finacea; Finevin; Skinorem; Skinoren; Acide azelaique; Acide azelaique [French]; Acido azelaico; Acido azelaico [Spanish]; Acidum azelaicum; Acidum azelaicum [Latin]; Anchoic acid; Azalaic Acid; Azelaic acid polyanhydride; Azelaic polyanhydride; Azelainic acid; Azleaic Acid; Heptanedicarboxylic acid; Lepargylic acid; NONANEDIOIC ACID; Nonanedioic acid; Polyazelaic anhydride; AZ1; Dicarboxylic acid C9; Emerox 1110; Emerox 1144; ZK 62498; A-9800; AGN-191861; Azelaic acid [USAN:INN]; Azelaic acid,technical grade; Azelex (TN); Finacea (TN); Finevin (TN); N-Nonanedioic acid; Nonanedioic acid, homopolymer; Poly(azelaic anhydride); SH-441; Skinoren (TN); ZK-62498; AZELAIC ACID, 95%; Azelaic acid (USAN/INN); Emery's L-110; 1,7-Heptanedicarboxylic acid; 1,9-Nonanedioic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Dermatologic Agents
|
||||||||||||||||||||||
| Affected Organisms |
Various aerobic and anaerobic microorganisms
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
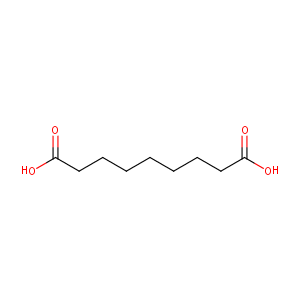 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 188.22 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7484). | ||||
|---|---|---|---|---|---|
| 2 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 3 | Inhibition of 5 alpha-reductase activity in human skin by zinc and azelaic acid. Br J Dermatol. 1988 Nov;119(5):627-32. | ||||
| 4 | Identification of specific ligands for orphan olfactory receptors. G protein-dependent agonism and antagonism of odorants. J Biol Chem. 2005 Mar 25;280(12):11807-15. doi: 10.1074/jbc.M411508200. Epub 2004 Dec 14. | ||||
| 5 | Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARgamma activation. Exp Dermatol. 2010 Sep;19(9):813-20. doi: 10.1111/j.1600-0625.2010.01107.x. Epub 2010 Jul 2. | ||||
| 6 | Azelaic acid decreases the fibrinolytic potential of cultured human melanoma cells in vitro. Cancer Lett. 1996 Jun 5;103(2):125-9. doi: 10.1016/0304-3835(96)04185-7. | ||||
