Details of the Drug
General Information of Drug (ID: DMIOFQE)
| Drug Name |
SOMATOSTATIN
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
51110-01-1; Somiaton; Panhibin; Aminopan; Somatostatin acetate; SRIF; Somatotropin release-inhibiting factor; Somatotropin release-inhibiting hormone; Growth hormone release-inhibiting factor; CCRIS 7071; EINECS 256-969-7; Growth hormone release-inhibiting hormone; AY 24910; Growth hormone release inhibiting factor; Somatostatin 1-14; Ala-Gly-Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys; NCGC00181005-01; 61950-59-2; [D-Trp8]-SOMATOSTATIN; SCHEMBL3046327
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Structure |
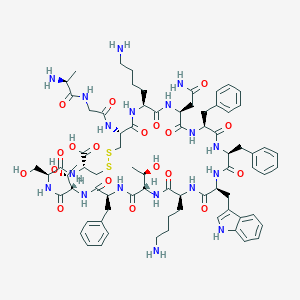 |
||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 1637.9 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -3.1 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 26 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 22 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 24 | ||||||||||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Acromegaly | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A60.0 | |||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||
References
