Details of the Drug
General Information of Drug (ID: DMIYLXU)
| Drug Name |
1-(4-ethyl-2,5-dimethoxyphenyl)propan-2-amine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
2,5-Dimethoxy-4-ethylamphetamine; 1-(4-ethyl-2,5-dimethoxyphenyl)propan-2-amine; 22004-32-6; DOET; CHEMBL8224; NCGC00247691-01; SCHEMBL1740704; DOET,(-); AC1L1D54; CTK4E8216; BDBM81965; HXJKWPGVENNMCC-UHFFFAOYSA-N; 4-Ethyl-2,5-dimethoxyamphetamine; PDSP2_000785; CAS_62066; PDSP1_001371; PDSP2_001355; PDSP1_000797; NSC_62066; DB01467; ACM22004326; 2,5-dimethoxy-4-ethylamphetamine (''DOEt''); L001150; Benzeneethanamine,4-ethyl-2,5-dimethoxy-a-methyl-; 1-(4-Ethyl-2,5-dimethoxyphenyl)-2-propanamine #; Phenethylamine, 4-ethyl-2,5-dimeth
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
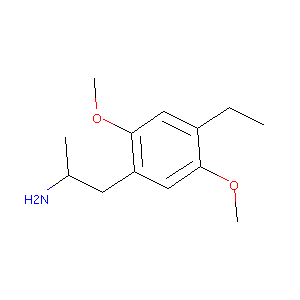 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 223.31 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
