Details of the Drug
General Information of Drug (ID: DMJKWPZ)
| Drug Name |
Cefpirome
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Cefpiroma; Cefpiroma [Spanish]; Cefpirome (INN); Cefpirome [INN:BAN]; Cefpiromum; Cefpiromum [Latin]; Cefrom; Cerfpirome; Keiten; Broact; CEFPIROME; S72Q2F09HY; cefpirome sulfate; cefpirome sulphate; (6R,7R)-7-{[(2Z)-2-(2-Amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-(6,7-dihydro-5H-cyclopenta[b]pyridinium-1-ylmethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate; 84957-29-9; C22H22N6O5S2; CHEBI:3503; HR 810; HR-810; NCGC00181339-01; UNII-S72Q2F09HY
|
|||||
| ATC Code | ||||||
| Structure |
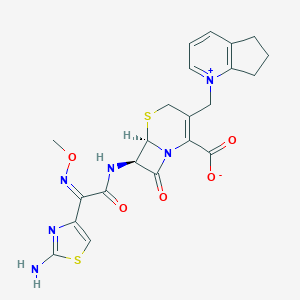 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 514.6 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 0.9 | |||||
| Rotatable Bond Count (rotbonds) | 6 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
