Details of the Drug
General Information of Drug (ID: DMLMOIJ)
| Drug Name |
Cyproterone acetate
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Cyprosterone acetate; Cyproteron acetate; Cyproteron-R acetate; Cyproterone 17-O-acetate; Cyproterone 17.alpha.-acetate; Cyproterone 17alpha-acetate; Cyproterone acetate [USAN:JAN]; Androcur; CYPROTERONE ACETATE; Cyproteroneacetate; SH 714; SH 80714; SH-714; 3'H-Cyclopropa(1,2)pregna-1,4,6-triene-3,20-dione, 17-(acetyloxy)-6-chloro-1,2-dihydro-, (1beta,2beta)-; 427-51-0; 4KM2BN5JHF; C24H29ClO4; CCRIS 4385; CHEBI:50743; CHEMBL139835; EINECS 207-048-3; HSDB 3592; MLS000859908; NSC 81430; NSC-81430; UNII-4KM2BN5JHF
|
|||||
| Affected Organisms |
Humans and other mammals
|
|||||
| Structure |
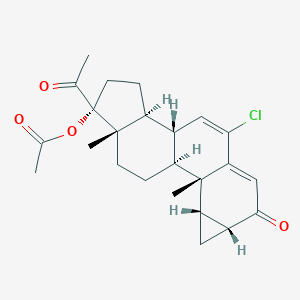 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 416.9 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 3.6 | |||||
| Rotatable Bond Count (rotbonds) | 3 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
