Details of the Drug
General Information of Drug (ID: DMN7Y9K)
| Drug Name |
Alpidem
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ALPIDEM; 82626-01-5; Ananxyl; Alpidemum [Latin]; UNII-I93SC245QZ; SL 80.0342-00; 2-(6-Chloro-2-(4-chlorophenyl)imidazo[1,2-a]pyridin-3-yl)-N,N-dipropylacetamide; 6-Chloro-2-(p-chlorophenyl)-N,N-dipropylimidazo(1,2-a)pyridine-3-acetamide; CHEMBL54349; I93SC245QZ; S-800342; NCGC00182850-01; 6-Chlor-2-(4-chlorphenyl)-N,N-dipropylimidazol(1,2-a)pyridin-3-acetamid; DSSTox_RID_83237; DSSTox_CID_28972; DSSTox_GSID_49046; Alpidemum; 6-Chloro-2-(4-chlorophenyl)-N,N-dipropyl-imidazo[1,2-a]pyridine-3-acetamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
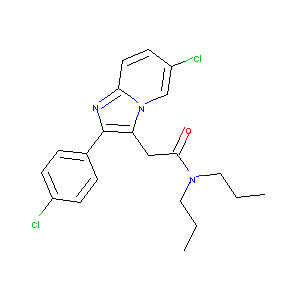 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 404.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
