Details of the Drug
General Information of Drug (ID: DMO2K0J)
| Drug Name |
Pyridoxal Phosphate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pyridoxal phosphate; pyridoxal phosphate; Codecarboxylase; pyridoxal 5-phosphate; 54-47-7; pyridoxal 5'-phosphate; Pyridoxal P; Pyromijin; Hairoxal; Vitazechs; Biosechs; Pyridoxal-5'-phosphate; Hiadelon; Pidopidon; Himitan; Phosphopyridoxal; Sechvitan; Pydoxal; Piodel; Pyridoxyl phosphate; Apolon B6; (4-formyl-5-hydroxy-6-methylpyridin-3-yl)methyl dihydrogen phosphate; HI-Pyridoxin; Pal-P; PYRIDOXAL-5-PHOSPHATE; pyridoxal-P; Phosphopyridoxal coenzyme; Vitahexin P; Hexermin P; Coenzyme B6; Pyridoxal monophosphate; Pyridoxaldehyde phosphate; Apolon B(sub 6); Biosechs; PLP; Pyrido; Phosphoridoxal coenzyme; Pyridoxal phosphate [JAN]; SRI 2392; VITAMIN B6 COMPLEX; Vitamin B6 phosphate; Hexermin-P; Pyridoxal 5-monophosphoric acid ester; Pyridoxal 5-phosphate; Pyridoxal phosphate (6CI); Pyridoxal-5P; Pyridoxal-P; Sechvitan, Vitahexin P; Vitahexin-P; Vitamin B6 phosphate (ester); Pyridoxal 5'-phosphate; Pyridoxal 5'-phosphate hydrate; Pyridoxal-5-Phosphate Hydrate; Pyridoxal-5-monophosphate; P-5'-P; Pyridoxal 5'-(dihydrogen phosphate); Pyridoxal 5'-phosphate monohydrate, vitamin B6; Pyridoxal 5'-phosphate monohydrate-Vitamin B6; Pyridoxal phosphate treated beta-lactoglobulin from bovine whey; Pyridoxal, 5-(dihydrogen phosphate); Pyridoxal, 5-(dihydrogenphosphate); Pyridoxal, 5-(dihydrogen phosphate) (8CI); Phosphoric acid mono-(4-formyl-5-hydroxy-6-methyl-pyridin-3-ylmethyl) ester; Isonicotinaldehyde, 3-hydroxy-5-(hydroxymethyl)-2-methyl-, 5-(dihydrogen phosphate); (4-Formyl-5-hydroxy-6-methyl(3-pyridyl))methyl dihydrogen phosphate; (4-formyl-5-hydroxy-6-methyl-3-pyridinyl)methyl dihydrogen phosphate; 2-Methyl-3-hydroxy-4-formyl-5-hydroxymethylpyridine-5-calcium phosphate; 2-Methyl-3-hydroxy-4-formyl-5-hydroxymethylpyridine-5-calcium phosphate trihydrate; 2-Methyl-3-hydroxy-4-formyl-5-pyridylmethylphosphoric acid; 3-Hydroxy-2-methyl-5-((phosphonooxy)methyl)-4-pyridinecarboxaldehyde; 3-Hydroxy-5-(hydroxymethyl)-2-methylisonicotinaldehyde 5-phosphate; 3-hydroxy-2-methyl-5-[(phosphonooxy)methyl]-4-pyridinecarboxaldehyde; 4-Formyl-5-hydroxy-6-methyl-pyridin-3-yl)methoxyphosphonic acid; 4-Pyridinecarboxaldehyde, 3-hydroxy-2-methyl-5-[(phosphonooxy)methyl]-(9CI); Lepirudine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Dietary supplement
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
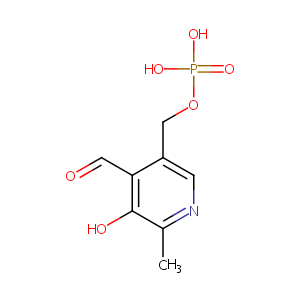 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 247.14 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
