Details of the Drug
General Information of Drug (ID: DMOBLGK)
| Drug Name |
Tromethamine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Trometamol; 77-86-1; TROMETHAMINE; Tris; Tris(Hydroxymethyl)aminomethane; Tham; Trisamine; 2-Amino-2-(hydroxymethyl)-1,3-propanediol; Trizma; Tris buffer; Tris base; Tromethane; Trisaminol; Talatrol; Trispuffer; Trisamin; Pehanorm; Tutofusin tris; Tris-steril; Apiroserum Tham; Addex-tham; Tris-base; Tris, free base; Trimethylolaminomethane; 1,3-Propanediol, 2-amino-2-(hydroxymethyl)-; Tris Amino; Aminotrimethylolmethane; Aminotris(hydroxymethyl)methane; THAM-E; Tris (buffering agent); Tris(Hydroxymethyl)Aminomethane
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
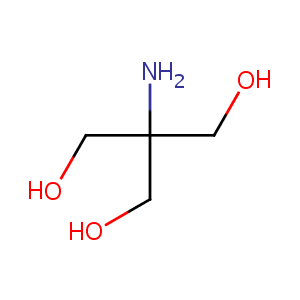 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 121.14 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Acidosis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5C73 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Tromethamine (Comorbidity)
|
|||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7328). | ||||
|---|---|---|---|---|---|
| 2 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | ||||
| 3 | Berg KJ "Acute effects of acetylsalicylic acid in patients with chronic renal insufficiency." Eur J Clin Pharmacol 11 (1977): 111-6. [PMID: 837963] | ||||
