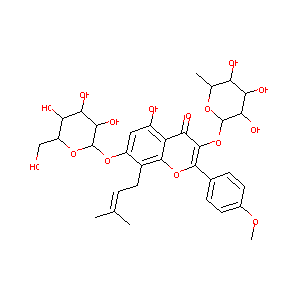
| Synonyms |
Icariin; 489-32-7; Ieariline; UNII-VNM47R2QSQ; CHEBI:78420; VNM47R2QSQ; Icariine; 3-((6-Deoxymannopyranosyl)oxy)-7-(glucopyranosyloxy)-5-hydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-butenyl)-4H-1-benzopyran-4-one; Icarin; 5-hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-7-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-3-(((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one; 4H-1-Benzopyran-4-one, 3-((6-deoxy-alpha-L-mannopyranosyl)oxy)-7-(be
|
| Chemical Identifiers |
- Formula
- C33H40O15
- IUPAC Name
5-hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxychromen-4-one - Canonical SMILES
-
C[C@H]1[C@@H]([C@H]([C@H]([C@@H](O1)OC2=C(OC3=C(C2=O)C(=CC(=C3CC=C(C)C)O[C@H]4[C@@H]([C@H]([C@@H]([C@H](O4)CO)O)O)O)O)C5=CC=C(C=C5)OC)O)O)O
- InChI
-
InChI=1S/C33H40O15/c1-13(2)5-10-17-19(45-33-28(42)26(40)23(37)20(12-34)46-33)11-18(35)21-24(38)31(48-32-27(41)25(39)22(36)14(3)44-32)29(47-30(17)21)15-6-8-16(43-4)9-7-15/h5-9,11,14,20,22-23,25-28,32-37,39-42H,10,12H2,1-4H3/t14-,20+,22-,23+,25+,26-,27+,28+,32-,33+/m0/s1
- InChIKey
-
TZJALUIVHRYQQB-XLRXWWTNSA-N
|