Details of the Drug
General Information of Drug (ID: DMP56WJ)
| Drug Name |
CF102
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
2-Cl-IB-Meca; 163042-96-4; Cl-IB-MECA; Namodenoson; C-Ibza-MU; CF-102; CI-IB-MECA; (2S,3S,4R,5R)-5-(2-Chloro-6-((3-iodobenzyl)amino)-9H-purin-9-yl)-3,4-dihydroxy-N-methyltetrahydrofuran-2-carboxamide; UNII-Z07JR07J6C; 2Cl-IB-MECA; 1-[2-CHLORO-6-[[(3-IODOPHENYL)METHYL]AMINO]-9H-PURIN-9-YL]-1-DEOXY-N-METHYL-BETA-D-RIBOFURANURONAMIDE; CHEMBL431733; Z07JR07J6C; CF102; 2-Chloro-N(6)-(3-iodobenzyl)adenosine-5'-N-methyluronamide
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
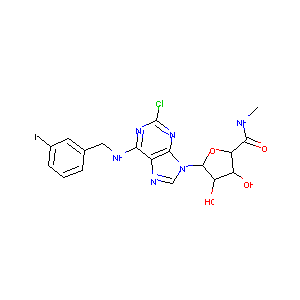 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 544.7 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
