Details of the Drug
General Information of Drug (ID: DMP62L5)
| Drug Name |
Resiniferatoxin
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Reciniferatoxin; UNII-A5O6P1UL4I; A5O6P1UL4I; 57444-62-9; RTX; [3H]resiniferatoxin; 6,7-Deepoxy-6,7-didehydro-5-deoxy-21-dephenyl-21-(phenylmethyl)daphnetoxin 20-(3-hydroxy-5-methoxybenzeneacetate); CHEMBL448382; resinferatoxin; Benzeneacetic acid, 4-hydroxy-3-methoxy-, ((2S,3aR,3bS,6aR,9aR,9bR,10R,11aR)-3a,3b,6,6a,9a,10,11,11a-octahydro-6a-hydroxy-8,10-dimethyl-11a-(1-methylethenyl)-7-oxo-2-(phenylmethyl)-7H-2,9b-epoxyazuleno(5,4-e)-1,3-benzodioxol-5-yl)methyl ester; Resiniferatoxin(RTX); CHEMBL17976; RTX
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
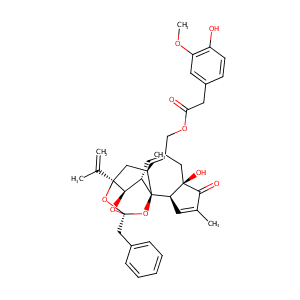 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 628.7 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.5 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
