Details of the Drug
General Information of Drug (ID: DMP9N85)
| Drug Name |
Menadiol sodium diphosphate
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Kappadione; Synkavit; Procoagulo; Synkavite; Thylokay; Kipca water soluble; Synkayvite; Menadiol sodium diphosphate; Menadione sodium phosphate; Sodium menadione diphosphate; Menadiol Sodium Diphosphate Anhydrous; UNII-R2L46WE615; Menadiol tetrasodium diphosphate; EINECS 205-012-1; Menadione diphosphate tetrasodium salt; 1:4-Naphthaquinol-bisdisodium phosphate; 131-13-5; Menadiol sodium diphosphate anhydrous [USAN]; R2L46WE615; 2-Methyl-1,4-naphthaquinol bis(disodium phosphate); Tetrasodium 2-methyl-1,4-naphthalenediol diphosp
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
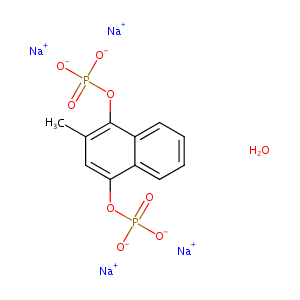 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 422.08 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||||||
| Rotatable Bond Count | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 8 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
