Details of the Drug
General Information of Drug (ID: DMP9TKE)
| Drug Name |
PTC299
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
6-(4-Fluorophenyl)-2,3-dihydro-5-(4-pyridinyl)imidazo(2,1-b)thiazole; skf-86002; 72873-74-6; Skf 86002; F 86002; F 86002-A(2); UNII-9R6QDF1UO7; 9R6QDF1UO7; CHEMBL313417; 5-(4-Pyridyl)-6-(4-fluorophenyl)-2,3-dihydroimidazo(2,1-b)-thiazole; 4-[6-(4-fluorophenyl)-2H,3H-imidazo[2,1-b][1,3]thiazol-5-yl]pyridine; 6-(4-fluorophenyl)-5-(4-pyridyl)-2,3-dihydroimidazo[2,1-b]thiazole; 6-(4-Fluorophenyl)-2,3-dihydro-5-(4-pyridyl)imidazo[2,1-b]thiazole; Imidazo(2,1-b)thiazole,; SK&F 86002
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
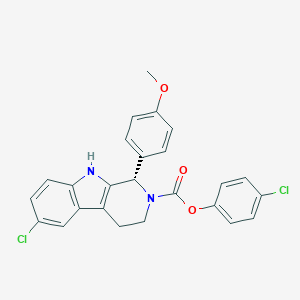 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 467.3 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.3 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Rheumatoid arthritis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | FA20 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
